# Let's talk about science: Pauli Exclusion Principle. # <div class="text-justify"> Hello, steemians today I would like to talk about one of the most important principles in the world of chemistry and physics, it is Pauli exclusion principle because it helps us to better understand the quantum world. # # Pauli Exclusion Principle. # <div class="pull-right"><center><img src="https://upload.wikimedia.org/wikipedia/commons/thumb/b/bd/Aufbau_Principle_1D.svg/450px-Aufbau_Principle_1D.svg.png" /><br/><em><sub><sub><a href="https://commons.wikimedia.org/wiki/File:Aufbau_Principle_1D.svg">Source</a></sub></sub></em></div> <br/><em><sub><sub><a href="URL LICENCIA">Public Domain</a></sub></sub></em></center></div> This principle states that basically, two equal particles cannot coexist in the same quantum state, that is, that both particles cannot be located in the same position or have the same speed, within the limits set by the uncertainty principle. > In short, there can not be two fermions with their four equal quantum numbers. Only two electrons can be inside the same orbital and with opposite spin. For example, electrons, which are part of the category of fermions, cannot be grouped one over another, because if we try to locate two electrons in the same orbit they will repel. <div class="pull-left"><center><img src="https://upload.wikimedia.org/wikipedia/commons/9/9a/FillingElectronDiagram.gif" /><br/><em><sub><sub><a href=" https://commons.wikimedia.org/wiki/File:FillingElectronDiagram.gif ">Source</a></sub></sub></em></div> <br/><em><sub><sub><a href="URL LICENCIA">Public Domain</a></sub></sub></em></center></div> It should be noted that Pauli's exclusion principle can only be applied to fermions, and not to bosons. This was verified through the realization of an experiment where two atoms were cooled one of Lithium-7 (boson) and another of Lithium-6 (fermion) until they reached temperatures close to those of absolute zero, in order to check that the boson cloud was compacted while the fermion cloud didn't experience significant changes due to the repulsion that Pauli establishes. # <hr> # The most important rule. # The most important rule that establishes Pauli's exclusion principle is that: > An atomic orbital can only have two electrons if the spins of both are opposite. These electrons of opposite spins are considered paired. Electrons of the same spin tend to be separated as much as possible. This tendency is the most important of the factors that determine the shapes and properties of molecules. # <hr> # Origin of the Pauli exclusion principle. # <div class="pull-right"><center><img src="https://upload.wikimedia.org/wikipedia/commons/e/ea/Wolfgang_Pauli.jpg" /><br/><em><sub><sub><a href="https://commons.wikimedia.org/wiki/File:Wolfgang_Pauli.jpg">Source</a></sub></sub></em></div> <br/><em><sub><sub><a href="URL LICENCIA">Public Domain</a></sub></sub></em></center></div> > The Pauli exclusion principle was developed by the Austrian physicist Wolfgang Ernst Pauli in 1925 However, before Pauli came to the formulation of this principle, first formulated another and it is the principle of antisymmetry, in which theorize that: > The total wave function of a group of electrons must be anti-symmetric with respect to the exchange of electrons. While for particles with integer spin (bosons), the wave function must be symmetric. This principle of anti-symmetry made Pauli formulate the principle of exclusion, in which it was established, as I mentioned above, that two electrons with the same set of quantum numbers cannot exist, that is, if two electrons have the same spatial part and spin. # <hr> # Importance of Pauli's exclusion principle. # According to Pauli exclusion principle, in an atomic type orbital, which is represented by the quantum numbers **n, l, and ml**, there must be two electrons; one of them with a positive spin +1/2 and another with its opposite spin negative -1/2. So basically, each of the types of orbitals can only have 2 electrons as maximums, and with spins of opposite signs. These electrons must also have equal quantum numbers, and will only differ in the quantum number **ms** (spin). <div class="pull-right"><center><img src="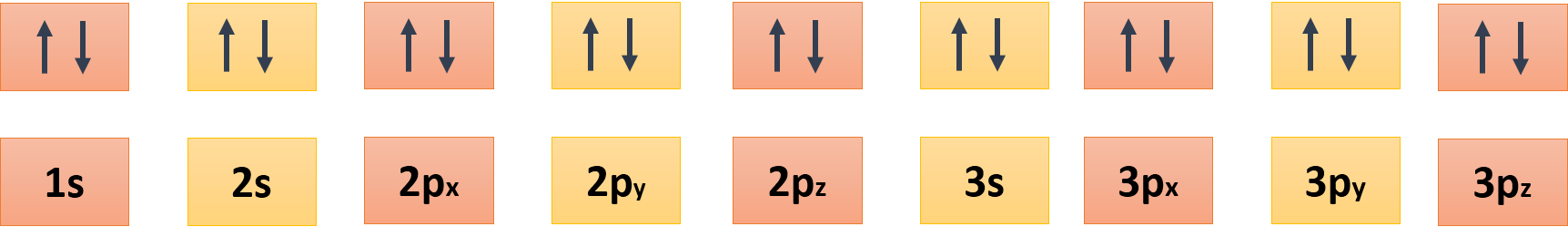" /><br/><em><sub><sub><a href="URL FUENTE DE LA IMAGEN">Made By me</a></sub></sub></em></div> <br/><em><sub><sub><a href="URL LICENCIA">@josalarcon2</a></sub></sub></em></center></div> To understand it better, when we distribute the electrons by layers, we represent an orbital with an upward arrow and another downward, which will indicate that there are two electrons in this orbital, but that they have spins of different signs. The presence of the Pauli exclusion principle is one of the main characteristics that make possible the distinction between what we consider as matter, photons and gravitons. The matter is made of fermions (protons, neutrons, and electrons), that is why they comply with the rules of the principle of Pauli's exclusion. Meanwhile, photons and gravitons are bosons, that is, they do not behave according to the principle of exclusion. # <hr> # Neutral atoms. # <div class="pull-left"><center><img src="https://upload.wikimedia.org/wikipedia/commons/thumb/8/84/Electron_shell_002_Helium_-_no_label.svg/600px-Electron_shell_002_Helium_-_no_label.svg.png" /><br/><em><sub><sub><a href="https://commons.wikimedia.org/wiki/File:Electron_shell_002_Helium_-_no_label.svg">Source</a></sub></sub></em></div> <br/><em><sub><sub><a href="https://creativecommons.org/licenses/by-sa/2.0/uk/deed.en">CC BY-SA 2.0</a></sub></sub></em></center></div> An electrically neutral atom has in its nucleus a number of articulated electrons (bounds) equal to the number of protons. Since electrons are fermions, and Pauli's exclusion principle forbids them from occupying the same quantum state. For example, suppose we have a neutral helium atom with two articulated electrons. These two electrons are capable of taking the lowest state of energy (E1), but as long as their spins are opposite. So that does not violate Pauli's principle of exclusion because the spins are part of the quantum state of the electron since these electrons are located in different quantum states. <div class="pull-right"><center><img src="https://upload.wikimedia.org/wikipedia/commons/thumb/a/ae/Electron_shell_003_Lithium_-_no_label.svg/600px-Electron_shell_003_Lithium_-_no_label.svg.png" /><br/><em><sub><sub><a href="https://commons.wikimedia.org/wiki/File:Electron_shell_003_Lithium_-_no_label.svg">Source</a></sub></sub></em></div> <br/><em><sub><sub><<a href="https://creativecommons.org/licenses/by-sa/2.0/uk/deed.en">CC BY-SA 2.0</a></sub></sub></em></center></div> On the other hand, neutral lithium atoms have three articulated electrons. Where two of its electrons are able to be located in the E1 state, but the third will always occupy the highest energy state (E2). In a similar way, it happens with the successive elements that produce increasingly more expensive coatings. Pauli's exclusion principle also explains the stability of the atomic orbitals, as well as the pressure that degenerate matter performs. # <hr> # References # > * [Pauli exclusion principle - Wikipedia](https://en.wikipedia.org/wiki/Pauli_exclusion_principle) > * [SPIN AND THE PAULI EXCLUSION PRINCIPLE - The physics of the universe](https://www.physicsoftheuniverse.com/topics_quantum_spin.html) > * [Pauli Exclusion Principle - Hyperphysics](http://hyperphysics.phy-astr.gsu.edu/hbase/pauli.html) > * [Pauli exclusion principle - Britannica](https://www.britannica.com/science/Pauli-exclusion-principle) > * [Quantum Numbers, Atomic Orbitals, and Electron Configurations](https://www.angelo.edu/faculty/kboudrea/general/quantum_numbers/Quantum_Numbers.htm) > * [How important is the Pauli exclusion principle in the distribution of particles on energy levels - Stack Exchange](https://physics.stackexchange.com/questions/240408/how-important-is-the-pauli-exclusion-principle-in-the-distribution-of-particles?utm_medium=organic&utm_source=google_rich_qa&utm_campaign=google_rich_qa) > * [Pauli’s Exclusion Principle: The Origin and Validation of a Scientific Principle, Michaela Massimi - Cambridge](ftp://nozdr.ru/biblio/kolxo3/P/PQm/Massimi%20M.%20Pauli%23s%20exclusion%20principle%20(CUP,%202005)(ISBN%200521839114)(227s)_PQm_.pdf) # <hr> <center> # SteemSTEM # **SteemSTEM** is a community-driven project that now runs on Steemit for more than a year. We seek to build a community of science lovers on Steemit and to promote well written/informative Science Technology Engineering and Mathematics (STEM) postings in order to make Steemit a place for fascinating STEM content. # ***So if you want to read more scientific articles of good quality, visit the hashtag #steemstem and scienceON.*** #  # **Disclaimer:** **[1] All the content exposed in this post is a compilation of different sources besides my knowledge in the subject.** **[2] All the images used are correctly labeled for reuse.** </center> </div>
| author | josalarcon2 |
|---|---|
| permlink | let-s-talk-about-science-pauli-exclusion-principle |
| category | steemstem |
| json_metadata | "{"community":"busy","app":"busy/2.4.0","format":"markdown","users":["josalarcon2"],"links":["https://commons.wikimedia.org/wiki/File:Aufbau_Principle_1D.svg","https://URL LICENCIA","https://commons.wikimedia.org/wiki/File:FillingElectronDiagram.gif","https://URL LICENCIA","https://commons.wikimedia.org/wiki/File:Wolfgang_Pauli.jpg","https://URL LICENCIA","https://URL FUENTE DE LA IMAGEN","https://URL LICENCIA","https://commons.wikimedia.org/wiki/File:Electron_shell_002_Helium_-_no_label.svg","https://creativecommons.org/licenses/by-sa/2.0/uk/deed.en"],"image":["https://steemitimages.com/0x0/https://upload.wikimedia.org/wikipedia/commons/thumb/b/bd/Aufbau_Principle_1D.svg/450px-Aufbau_Principle_1D.svg.png","https://steemitimages.com/0x0/https://upload.wikimedia.org/wikipedia/commons/9/9a/FillingElectronDiagram.gif","https://steemitimages.com/0x0/https://upload.wikimedia.org/wikipedia/commons/e/ea/Wolfgang_Pauli.jpg","https://steemitimages.com/0x0/","https://steemitimages.com/0x0/https://upload.wikimedia.org/wikipedia/commons/thumb/8/84/Electron_shell_002_Helium_-_no_label.svg/600px-Electron_shell_002_Helium_-_no_label.svg.png","https://steemitimages.com/0x0/https://upload.wikimedia.org/wikipedia/commons/thumb/a/ae/Electron_shell_003_Lithium_-_no_label.svg/600px-Electron_shell_003_Lithium_-_no_label.svg.png","https://steemitimages.com/0x0/https://steemitimages.com/DQmY5sQNXqo9QtNEpNeNzPVn8LsecTGrGUpWr4jHDqpZG8i/steemlogo.jpg"],"tags":["steemstem","science","physics","davinci-times","busy"]}" |
| created | 2018-04-12 12:55:42 |
| last_update | 2018-04-12 12:55:42 |
| depth | 0 |
| children | 3 |
| last_payout | 2018-04-19 12:55:42 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 1.826 HBD |
| curator_payout_value | 0.441 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 9,265 |
| author_reputation | 45,465,284,339,012 |
| root_title | "Let's talk about science: Pauli Exclusion Principle" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 49,670,567 |
| net_rshares | 494,160,882,153 |
| author_curate_reward | "" |
| voter | weight | wgt% | rshares | pct | time |
|---|---|---|---|---|---|
| pharesim | 0 | 78,348,635,249 | 0.02% | ||
| lafona-miner | 0 | 0 | 0% | ||
| hr1 | 0 | 52,044,952,419 | 0.02% | ||
| kushed | 0 | 0 | 0% | ||
| bue | 0 | 13,988,807,695 | 100% | ||
| justtryme90 | 0 | 0 | 0% | ||
| anwenbaumeister | 0 | 0 | 0% | ||
| mammasitta | 0 | 19,755,007,658 | 5% | ||
| liberosist | 0 | 0 | 0% | ||
| lemouth | 0 | 0 | 0% | ||
| rjbauer85 | 0 | 0 | 0% | ||
| anarchyhasnogods | 0 | 0 | 0% | ||
| lamouthe | 0 | 1,177,893,482 | 5% | ||
| meerkat | 0 | 0 | 0% | ||
| curie | 0 | 0 | 0% | ||
| hendrikdegrote | 0 | 0 | 0% | ||
| steemstem | 0 | 0 | 0% | ||
| dashfit | 0 | 114,456,221 | 0.44% | ||
| sethroot | 0 | 92,601,397 | 0.08% | ||
| busy.org | 0 | 77,951,408,128 | 2.53% | ||
| foundation | 0 | 0 | 0% | ||
| the-devil | 0 | 0 | 0% | ||
| thevenusproject | 0 | 2,774,023,084 | 5% | ||
| dna-replication | 0 | 0 | 0% | ||
| boynashruddin | 0 | 327,247,503 | 5% | ||
| hagbardceline | 0 | 145,599,298,544 | 100% | ||
| pacokam8 | 0 | 83,823,330 | 0.22% | ||
| borislavzlatanov | 0 | 428,716,917 | 5% | ||
| michelios | 0 | 1,027,327,545 | 0.08% | ||
| jamhuery | 0 | 0 | 0% | ||
| kryzsec | 0 | 0 | 0% | ||
| markangeltrueman | 0 | 332,503,483 | 0.13% | ||
| gktown | 0 | 84,300,855 | 0.55% | ||
| resteemable | 0 | 638,879,318 | 1.1% | ||
| tantawi | 0 | 81,813,289 | 0.89% | ||
| samminator | 0 | 1,923,817,340 | 5% | ||
| minnowsupport | 0 | 24,713,012,242 | 0.5% | ||
| locikll | 0 | 0 | 0% | ||
| dber | 0 | 0 | 0% | ||
| mahdiyari | 0 | 337,950,061 | 0.44% | ||
| aboutyourbiz | 0 | 210,930,121 | 0.89% | ||
| kerriknox | 0 | 0 | 0% | ||
| alexander.alexis | 0 | 446,520,285 | 1.5% | ||
| howtostartablog | 0 | 392,067,762 | 0.04% | ||
| blessing97 | 0 | 0 | 0% | ||
| slickhustler007 | 0 | 50,357,649 | 0.44% | ||
| ertwro | 0 | 0 | 0% | ||
| apoloo1 | 0 | 3,164,743,452 | 10% | ||
| coloringiship | 0 | 167,064,692 | 0.04% | ||
| juanjdiaz89 | 0 | 211,762,418 | 5% | ||
| thinknzombie | 0 | 1,564,606,213 | 0.44% | ||
| nitesh9 | 0 | 0 | 0% | ||
| himal | 0 | 0 | 0% | ||
| abigail-dantes | 0 | 0 | 0% | ||
| leczy | 0 | 326,272,562 | 5% | ||
| ovij | 0 | 651,571,875 | 5% | ||
| suravsingh | 0 | 0 | 0% | ||
| joseg | 0 | 61,359,315 | 3% | ||
| alexzicky | 0 | 0 | 0% | ||
| mountain.phil28 | 0 | 2,527,162,028 | 25% | ||
| zest | 0 | 3,440,094,481 | 10% | ||
| felixrodriguez | 0 | 120,199,427 | 2.5% | ||
| pearlumie | 0 | 1,879,559,543 | 5% | ||
| tormiwah | 0 | 235,019,674 | 1.5% | ||
| gabox | 0 | 174,280,073 | 0.04% | ||
| infinitelearning | 0 | 116,612,351 | 2% | ||
| massivevibration | 0 | 7,061,290,757 | 5% | ||
| onartbali | 0 | 1,078,737,288 | 5% | ||
| spotlight | 0 | 453,772,139 | 0.55% | ||
| clweeks | 0 | 60,517,000 | 0.44% | ||
| ksolymosi | 0 | 967,778,999 | 5% | ||
| simplifylife | 0 | 755,030,893 | 2.5% | ||
| jordanx2 | 0 | 59,409,806 | 0.44% | ||
| mayowadavid | 0 | 414,563,978 | 2.5% | ||
| imamalkimas | 0 | 59,357,096 | 0.89% | ||
| zeeshan003 | 0 | 0 | 0% | ||
| enzor | 0 | 85,444,733 | 2.5% | ||
| bobdos | 0 | 288,349,353 | 0.08% | ||
| carloserp-2000 | 0 | 0 | 0% | ||
| pangoli | 0 | 0 | 0% | ||
| rachelsmantra | 0 | 0 | 0% | ||
| gra | 0 | 0 | 0% | ||
| katerinaramm | 0 | 17,216,037,937 | 50% | ||
| aboutcoolscience | 0 | 0 | 0% | ||
| skycae | 0 | 241,085,579 | 0.89% | ||
| kenadis | 0 | 0 | 0% | ||
| amavi | 0 | 0 | 0% | ||
| robotics101 | 0 | 200,033,929 | 4% | ||
| gentleshaid | 0 | 0 | 0% | ||
| zalandir | 0 | 133,117,718 | 0.17% | ||
| cgbartow | 0 | 169,628,209 | 2.5% | ||
| sco | 0 | 182,112,712 | 0.25% | ||
| adetola | 0 | 196,456,215 | 5% | ||
| rharphelle | 0 | 1,836,611,443 | 100% | ||
| dysfunctional | 0 | 222,567,253 | 2.5% | ||
| whileponderin | 0 | 107,948,017 | 5% | ||
| bennettitalia | 0 | 96,225,105 | 0.04% | ||
| hadji | 0 | 0 | 0% | ||
| sakura1012 | 0 | 0 | 0% | ||
| echavez82 | 0 | 52,182,857 | 10% | ||
| terrylovejoy | 0 | 554,886,418 | 1.25% | ||
| wisewoof | 0 | 109,841,084 | 0.44% | ||
| futuremind | 0 | 246,486,804 | 0.89% | ||
| ndnthor | 0 | 51,985,157 | 10% | ||
| rionpistorius | 0 | 105,771,665 | 2.5% | ||
| josalarcon2 | 0 | 966,929,156 | 100% | ||
| ideas-abstractas | 0 | 749,487,699 | 100% | ||
| deutsch-boost | 0 | 363,374,235 | 20% | ||
| warpedpoetic | 0 | 74,443,241 | 0.89% | ||
| franklinjgc | 0 | 58,269,420 | 10% | ||
| dexterdev | 0 | 0 | 0% | ||
| ejrangel | 0 | 612,663,353 | 100% | ||
| ugonma | 0 | 72,451,024 | 5% | ||
| drkomoo | 0 | 61,750,060 | 5% | ||
| benleemusic | 0 | 1,341,675,009 | 0.04% | ||
| giovaabbatichio | 0 | 636,068,235 | 100% | ||
| chimtivers96 | 0 | 61,756,761 | 0.89% | ||
| positiveninja | 0 | 112,162,017 | 0.44% | ||
| davinci.witness | 0 | 0 | 0% | ||
| theunlimited | 0 | 52,225,947 | 10% | ||
| pseudojew | 0 | 84,046,426 | 5% | ||
| victorcovrig | 0 | 69,233,427 | 1% | ||
| de-stem | 0 | 1,768,724,699 | 4% | ||
| serylt | 0 | 835,990,652 | 4% | ||
| spaghettiscience | 0 | 13,224,931,550 | 50% | ||
| saracampero | 0 | 85,997,060 | 20% | ||
| xposed | 0 | 2,026,998,420 | 10% | ||
| davinci.polyglot | 0 | 0 | 0% | ||
| davinci.times | 0 | 0 | 0% | ||
| itastem | 0 | 0 | 0% | ||
| niouton | 0 | 128,961,710 | 0.17% | ||
| biomimi | 0 | 198,852,227 | 40% |
Very well written post, as a chemistry student i appreciate the topic :) keep up the good work!!
| author | akeelsingh |
|---|---|
| permlink | re-josalarcon2-let-s-talk-about-science-pauli-exclusion-principle-20180413t170227803z |
| category | steemstem |
| json_metadata | {"tags":["steemstem"],"app":"steemit/0.1"} |
| created | 2018-04-13 17:02:24 |
| last_update | 2018-04-13 17:02:24 |
| depth | 1 |
| children | 0 |
| last_payout | 2018-04-20 17:02:24 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 96 |
| author_reputation | 2,407,869,811,389 |
| root_title | "Let's talk about science: Pauli Exclusion Principle" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 49,886,984 |
| net_rshares | 3,922,714,357 |
| author_curate_reward | "" |
| voter | weight | wgt% | rshares | pct | time |
|---|---|---|---|---|---|
| akeelsingh | 0 | 3,922,714,357 | 100% |
<p>Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josalarcon2 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the <a href="https://discord.gg/HYj4yvw"> Peace, Abundance, and Liberty Network (PALnet) Discord Channel</a>. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.</p> <p>If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: <a href="https://v2.steemconnect.com/sign/delegateVestingShares?delegator=&delegatee=minnowsupport&vesting_shares=102530.639667%20VESTS">50SP</a>, <a href="https://v2.steemconnect.com/sign/delegateVestingShares?delegator=&delegatee=minnowsupport&vesting_shares=205303.639667%20VESTS">100SP</a>, <a href="https://v2.steemconnect.com/sign/delegateVestingShares?delegator=&delegatee=minnowsupport&vesting_shares=514303.639667%20VESTS">250SP</a>, <a href="https://v2.steemconnect.com/sign/delegateVestingShares?delegator=&delegatee=minnowsupport&vesting_shares=1025303.639667%20VESTS">500SP</a>, <a href="https://v2.steemconnect.com/sign/delegateVestingShares?delegator=&delegatee=minnowsupport&vesting_shares=2053030.639667%20VESTS">1000SP</a>, <a href="https://v2.steemconnect.com/sign/delegateVestingShares?delegator=&delegatee=minnowsupport&vesting_shares=10253030.639667%20VESTS">5000SP</a>. <br><strong>Be sure to leave at least 50SP undelegated on your account.</strong></p>
| author | minnowsupport |
|---|---|
| permlink | re-let-s-talk-about-science-pauli-exclusion-principle-20180414t222744 |
| category | steemstem |
| json_metadata | "" |
| created | 2018-04-14 22:27:45 |
| last_update | 2018-04-14 22:27:45 |
| depth | 1 |
| children | 0 |
| last_payout | 2018-04-21 22:27:45 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 1,707 |
| author_reputation | 148,902,805,319,183 |
| root_title | "Let's talk about science: Pauli Exclusion Principle" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 50,098,956 |
| net_rshares | 0 |
**Your Post Has Been Featured on @Resteemable!** <br> Feature any Steemit post using resteemit.com! <br> **How It Works:** <br> 1. Take Any Steemit URL <br> 2. Erase `https://` <br> 3. Type `re`<br> Get Featured Instantly & Featured Posts are voted every 2.4hrs <br>[Join the Curation Team Here](https://goo.gl/forms/4sr0InoTxcyPRQSj2) | [Vote Resteemable for Witness](https://v2.steemconnect.com/sign/account-witness-vote?witness=resteemable&approve=1)
| author | resteemable |
|---|---|
| permlink | re-resteemable-let-s-talk-about-science-pauli-exclusion-principle-20180412t152559048z |
| category | steemstem |
| json_metadata | "" |
| created | 2018-04-12 15:25:57 |
| last_update | 2018-04-12 15:25:57 |
| depth | 1 |
| children | 0 |
| last_payout | 2018-04-19 15:25:57 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 453 |
| author_reputation | 711,299,530,826 |
| root_title | "Let's talk about science: Pauli Exclusion Principle" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 49,693,665 |
| net_rshares | 0 |
 hiveblocks
hiveblocks