Hello hivers across the world, hope you're all doing good and great?
Do you like to know more about isomerism in chemistry and its various types, if yes this post is meant for you.
The principal phenomenon that makes carbon a most unique element among all others on the periodic table is that of isomerism. This is the existence of two or several compounds having the same molecular weight, same elemental compositions but differing in the arrangement of constituent elements. Isomerism is seldom encountered in inorganic compounds but it has a widespread occurrence in the chemistry of organic compounds. To drive home the point, the formula C6H14 can give rise to five compounds while the formula C20H42 accommodates 366,319 different compounds which are isomeric to one another. There is no rule or formula to determine how many isomers a molecular formula will give rise to, but **as the carbon chain increases so also does the number of isomers.** This phenomenon compels us to look beyond the molecular formula into the details of the inner structure of organic molecules to unearth any possible arrangement that can result into another isomer. For clarity, the different bond orders that constitute different classes of isomerism are treated below.
Isomerism can be divided into two major groups; they are **structural isomerism** and **stereoisomerism**.
**STRUCTURAL ISOMERISM**
In structural isomerism, two or more compounds share the same composition but there is difference in the spatial arrangement of the atoms. Structural isomerism can be subdivided into chain isomerism, positional isomerism, functional group isomerism, chain-cycle isomerism, metamerism and tautomerism.
* **Positional Isomerism**: In positional isomerism, while the carbon chain remains the same, the functional groups shifts position. C4H8 has two of such isomers.
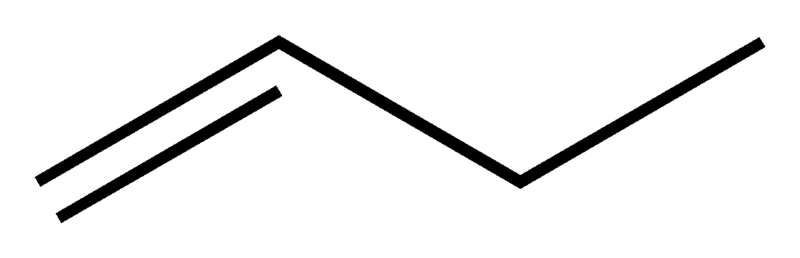
<center>[But-1-ene: sourced from wikimedia.org](https://upload.wikimedia.org/wikipedia/commons/9/9c/But-1-ene-2D-skeletal.png)</center>
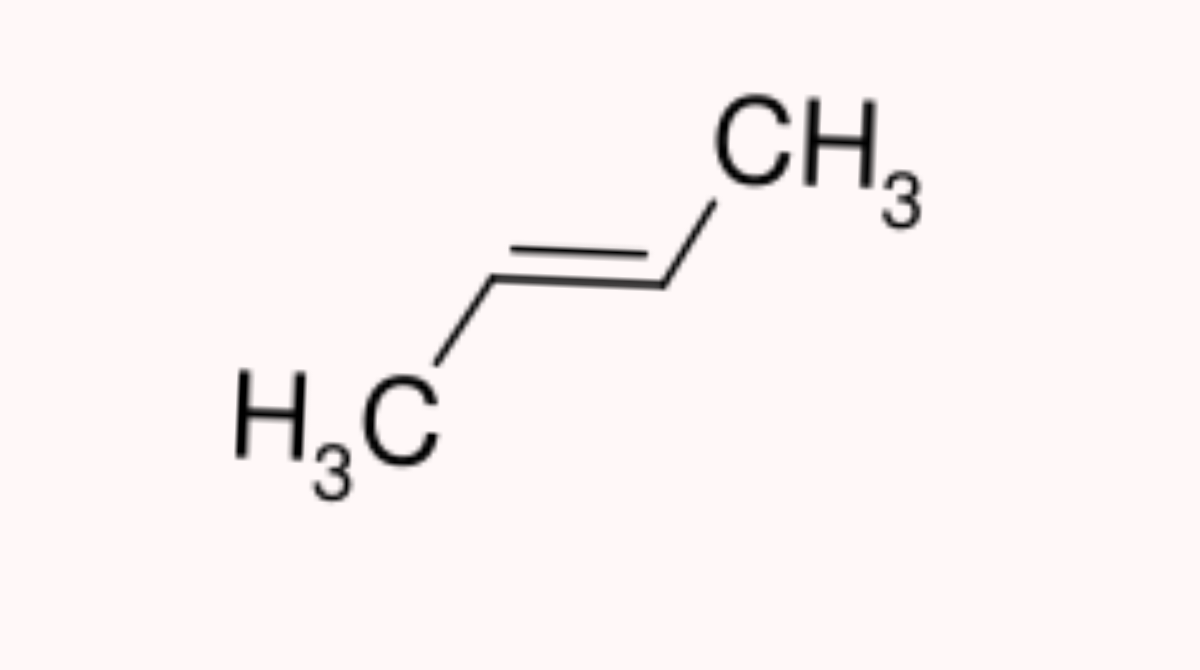
<center>[But-2-ene: sourced from wikimedia.org](https://upload.wikimedia.org/wikipedia/commons/d/dc/But-2-ene.svg)</center>
As seen above, the molecular formula is the same for both compounds(C4H8) but the structural arrangement is different which makes the name different.
* **Functional group isomerism**: There occurs frequently, isomers which belong to different functional groups, such occurrence is termed functional group isomerism. Propanal and acetone (propanone) share the same formula C3H6O but belong to two functional groups.
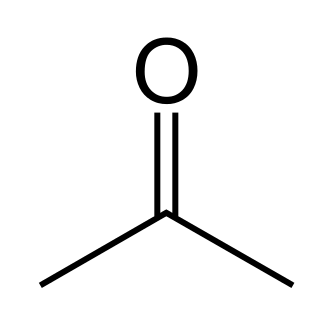
<center>[Propanone: sourced from wikimedia.org](https://upload.wikimedia.org/wikipedia/commons/f/fb/Acetone-2D-skeletal.svg)</center>
<center>[Propanal: sourced from wikimedia.org](https://upload.wikimedia.org/wikipedia/commons/d/d7/Propanal_propenol_tautomery.svg)</center>
* **Chain-cycle isomerism**: To the aforementiined groups of structural isomers must be added to the isomerism that occurs between multiple bonded straight chains and cyclic hydrocarbons. The structure C6H12 can give rise to alkenes as well as cyclic alkanes.
-hex-3-ene_200.svg.png)
<center>[Hex-3-ene: sourced from wikimedia.org](
https://upload.wikimedia.org/wikipedia/commons/1/1a/%28E%29-hex-3-ene_200.svg)</center>
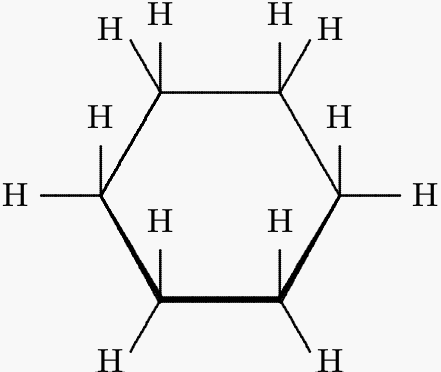
<center>[Cyclohexane: sourced from wikimedia.org](https://upload.wikimedia.org/wikipedia/commons/2/25/Cyclohexane-structure.png)</center>
* **Metamerism**: Metamers are members of the same homologous series but different radicals are attached to the same functional group as it may occur in ethers, ketones or amines.
For example, there are three ether isomers having the formula C4H10O. They are **Methoxy propane**, **Ethoxyethane** and **2-Methoxy propane**.
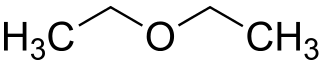
<center>[Ethoxyethane: sourced from wikimedia.org](https://upload.wikimedia.org/wikipedia/commons/f/fd/Ethoxyethane_200.svg)</center>
* **Tautomerism**: This is a phenomenon where a pair of isomers are in direct equilibrium with each other. In a basic medium, acetone exists in two enolate ions at equilibrium with each other.
**CONCLUSION**
Isomerism is a phenomenon where substances have the same molecular but differs in their structural arrangement. Isomerism is the phenomenon of the existence of isomers - the existence of different substances with the same molecular formulae; the interconversion of isomers.
### References
[Isomerism in Organic Chemistry](https://www.compoundchem.com/2014/05/22/typesofisomerism/)
[Types of isomerism](https://byjus.com/chemistry/isomerism/)
| author | sirpee6 |
|---|---|
| permlink | isomerism-in-organic-compounds |
| category | hive-196387 |
| json_metadata | {"tags":["hive-196387","steemstem","stemgeeks","science","chemistry","stem","discovery-it","neoxian"],"image":["https://images.hive.blog/DQmaa6PSxmGDzQ9yX81GKTHwJeQYH1qPMBecUnDuQvMPT6A/800px-But-1-ene-2D-skeletal.png","https://images.hive.blog/DQmSn7cjQzc4VQiWANzKY25iMb68AwvXVD3QYbrsbV57pQi/Adobe_Post_20200803_1710150.729127613527377.png","https://images.hive.blog/DQmZFDKJ794Q4WAkdkY8An3ekGGVTgF2R5mPbthphH85nVy/Acetone-2D-skeletal.svg.png","https://images.hive.blog/DQmPdsguzDtG18xbsfCMSr7sDY3am1nQvdQ9YgmgenhofQD/Adobe_Post_20200804_1115050.4751029316342755.png","https://images.hive.blog/DQmWdLcqhrc12pftdA3VKB9sYz6kcUcvzp2XVsdicpKF5fh/(E)-hex-3-ene_200.svg.png","https://images.hive.blog/DQmbrCvfN5mfadUrc35hqbNHGzUkaCmqt7hMMxNxy52EEsf/Cyclohexane-structure.png","https://images.hive.blog/DQmaRH3h33rtftMtw77aQqYB8vy2FuRn6FsRjhmNSXgynNf/Ethoxyethane_200.svg.png","https://images.hive.blog/DQmZeFXU7kyyiHZs61GRDDTPaUE1ksJ3i4CADBBdrP16A8L/LEGEND_20200719_112017.gif"],"links":["https://upload.wikimedia.org/wikipedia/commons/9/9c/But-1-ene-2D-skeletal.png","https://upload.wikimedia.org/wikipedia/commons/d/dc/But-2-ene.svg","https://upload.wikimedia.org/wikipedia/commons/f/fb/Acetone-2D-skeletal.svg","https://upload.wikimedia.org/wikipedia/commons/d/d7/Propanal_propenol_tautomery.svg","https://upload.wikimedia.org/wikipedia/commons/1/1a/%28E%29-hex-3-ene_200.svg","https://upload.wikimedia.org/wikipedia/commons/2/25/Cyclohexane-structure.png","https://upload.wikimedia.org/wikipedia/commons/f/fd/Ethoxyethane_200.svg","https://www.compoundchem.com/2014/05/22/typesofisomerism/","https://byjus.com/chemistry/isomerism/"],"app":"hiveblog/0.1","format":"markdown"} |
| created | 2020-08-04 13:00:48 |
| last_update | 2020-08-04 13:00:48 |
| depth | 0 |
| children | 3 |
| last_payout | 2020-08-11 13:00:48 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 3.590 HBD |
| curator_payout_value | 3.557 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 5,610 |
| author_reputation | 11,551,141,671,698 |
| root_title | "ISOMERISM IN ORGANIC COMPOUNDS" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 98,887,968 |
| net_rshares | 17,088,934,222,468 |
| author_curate_reward | "" |
| voter | weight | wgt% | rshares | pct | time |
|---|---|---|---|---|---|
| enki | 0 | 9,286,080,932 | 0.05% | ||
| tombstone | 0 | 9,894,036,502 | 0.49% | ||
| chris4210 | 0 | 5,856,282,168 | 0.65% | ||
| eric-boucher | 0 | 2,714,207,396 | 0.65% | ||
| anwenbaumeister | 0 | 20,003,787 | 1.3% | ||
| roelandp | 0 | 262,643,151,055 | 5% | ||
| matt-a | 0 | 620,469,082 | 1.5% | ||
| gikitiki | 0 | 2,958,695,604 | 5.5% | ||
| arcange | 0 | 108,486,749,057 | 3% | ||
| raphaelle | 0 | 2,889,882,528 | 3% | ||
| djennyfloro | 0 | 1,282,943,470 | 10% | ||
| kpine | 0 | 1,698,139,058,761 | 7% | ||
| sc-steemit | 0 | 1,301,893,493 | 0.65% | ||
| celestine | 0 | 0 | 3% | ||
| lemouth | 0 | 133,206,579,950 | 7.5% | ||
| notconvinced | 0 | 4,708,687,685 | 10% | ||
| charlie777pt | 0 | 1,963,992,668 | 5% | ||
| alaqrab | 0 | 677,414,537 | 0.65% | ||
| lamouthe | 0 | 783,810,185 | 10% | ||
| uwelang | 0 | 7,029,689,973 | 0.65% | ||
| holoz0r | 0 | 293,473,006 | 1.95% | ||
| tfeldman | 0 | 1,222,675,822 | 0.91% | ||
| mcsvi | 0 | 113,024,175,803 | 50% | ||
| lk666 | 0 | 616,486,905 | 0.65% | ||
| cnfund | 0 | 598,322,069 | 0.65% | ||
| curie | 0 | 201,837,869,558 | 1.3% | ||
| siniceku | 0 | 2,036,001,935 | 100% | ||
| modernzorker | 0 | 593,351,067 | 0.91% | ||
| techslut | 0 | 21,235,384,118 | 4% | ||
| hendrikdegrote | 0 | 16,717,787,800 | 1.3% | ||
| steemstem | 0 | 1,034,706,613,824 | 10% | ||
| zorg67 | 0 | 613,303,723 | 100% | ||
| apsu | 0 | 759,837,459 | 0.45% | ||
| ethandsmith | 0 | 14,539,887,208 | 2.75% | ||
| valth | 0 | 1,179,186,177 | 5% | ||
| dna-replication | 0 | 2,793,771,503 | 10% | ||
| steemitboard | 0 | 2,591,861,104 | 2% | ||
| tabea | 0 | 7,248,973,906 | 5.5% | ||
| dhimmel | 0 | 132,688,987,053 | 2.5% | ||
| bloom | 0 | 37,193,369,956 | 10% | ||
| federacion45 | 0 | 1,416,878,414 | 0.65% | ||
| iansart | 0 | 2,048,574,039 | 0.65% | ||
| mobbs | 0 | 27,137,022,586 | 5% | ||
| jagged | 0 | 2,680,885,168 | 1.65% | ||
| activate.alpha | 0 | 4,403,115,969 | 0.97% | ||
| roomservice | 0 | 54,780,264,462 | 1.23% | ||
| bitrocker2020 | 0 | 661,668,341 | 0.06% | ||
| farizal | 0 | 1,942,510,291 | 6% | ||
| steempearls | 0 | 0 | 2.75% | ||
| yehey | 0 | 12,651,339,031 | 1.3% | ||
| helo | 0 | 8,621,045,548 | 5% | ||
| samminator | 0 | 7,137,216,500 | 5% | ||
| locikll | 0 | 490,630,566 | 2.6% | ||
| mahdiyari | 0 | 23,602,387,959 | 5% | ||
| lorenzor | 0 | 6,269,868,130 | 50% | ||
| firstamendment | 0 | 56,805,507,523 | 50% | ||
| alexander.alexis | 0 | 5,633,628,640 | 10% | ||
| techken | 0 | 3,350,823,082 | 5.5% | ||
| d-pend | 0 | 3,188,376,211 | 0.11% | ||
| gunthertopp | 0 | 5,672,998,755 | 0.13% | ||
| flatman | 0 | 731,297,462 | 1.3% | ||
| tattoodjay | 0 | 4,071,295,499 | 0.42% | ||
| minnowbooster | 0 | 1,939,738,451,846 | 20% | ||
| tsoldovieri | 0 | 973,493,943 | 5% | ||
| stayoutoftherz | 0 | 14,852,923,024 | 0.65% | ||
| abigail-dantes | 0 | 970,870,098 | 10% | ||
| macchiata | 0 | 5,671,013,655 | 5.5% | ||
| sciencevienna | 0 | 4,114,212,335 | 5.5% | ||
| joseg | 0 | 866,514,685 | 80% | ||
| investingpennies | 0 | 3,552,411,199 | 1.3% | ||
| iamphysical | 0 | 4,898,579,089 | 90% | ||
| felixrodriguez | 0 | 1,495,478,718 | 35% | ||
| valchiz | 0 | 1,407,923,489 | 10% | ||
| betterthanhome | 0 | 2,405,213,444 | 0.65% | ||
| revo | 0 | 5,138,886,358 | 1.3% | ||
| azulear | 0 | 3,152,622,716 | 100% | ||
| djlethalskillz | 0 | 3,424,890,183 | 5% | ||
| thelordsharvest | 0 | 2,088,873,414 | 1.3% | ||
| massivevibration | 0 | 9,441,737,432 | 5% | ||
| holbein81 | 0 | 11,612,249,785 | 1.1% | ||
| justinparke | 0 | 2,836,955,392 | 2% | ||
| yangoldberg | 0 | 2,139,478,830 | 1.3% | ||
| therealwolf | 0 | 454,218,997,774 | 1.65% | ||
| revisesociology | 0 | 1,524,421,662 | 0.13% | ||
| upme | 0 | 637,166,098 | 0.65% | ||
| braveboat | 0 | 2,348,190,202 | 8% | ||
| jlsplatts | 0 | 8,531,461,303 | 1% | ||
| meno | 0 | 10,562,220,534 | 0.65% | ||
| buttcoins | 0 | 904,530,616 | 0.26% | ||
| toocurious | 0 | 864,719,177 | 0.65% | ||
| carloserp-2000 | 0 | 16,105,757,761 | 100% | ||
| lays | 0 | 3,227,788,582 | 0.65% | ||
| fknmayhem | 0 | 3,002,679,819 | 7.26% | ||
| gra | 0 | 844,083,716 | 10% | ||
| coyotelation | 0 | 1,969,065,215 | 10% | ||
| omstavan | 0 | 7,214,729,168 | 100% | ||
| steveconnor | 0 | 781,876,334 | 0.65% | ||
| sankysanket18 | 0 | 10,031,781,314 | 5% | ||
| smartsteem | 0 | 99,915,764,205 | 1.65% | ||
| drmake | 0 | 695,623,520 | 0.65% | ||
| danile666 | 0 | 1,403,621,143 | 1.23% | ||
| amestyj | 0 | 7,420,742,263 | 100% | ||
| mhm-philippines | 0 | 1,947,260,935 | 0.65% | ||
| kcherukuri | 0 | 18,771,338,782 | 50% | ||
| kenadis | 0 | 2,599,547,258 | 10% | ||
| steempsych | 0 | 714,558,069 | 10% | ||
| robotics101 | 0 | 2,749,685,835 | 10% | ||
| sco | 0 | 7,701,592,929 | 6.6% | ||
| ennyta | 0 | 959,932,378 | 50% | ||
| vjap55 | 0 | 960,332,315 | 100% | ||
| eliaschess333 | 0 | 1,909,134,001 | 50% | ||
| intrepidphotos | 0 | 110,492,479,675 | 2% | ||
| hijosdelhombre | 0 | 2,273,478,643 | 2.5% | ||
| matheusggr | 0 | 10,476,088,049 | 5.5% | ||
| fragmentarion | 0 | 2,032,964,132 | 10% | ||
| utube | 0 | 634,910,934 | 1.3% | ||
| terrylovejoy | 0 | 4,018,302,441 | 4% | ||
| neneandy | 0 | 1,456,491,570 | 1.3% | ||
| videosteemit | 0 | 956,768,583 | 1.3% | ||
| stemng | 0 | 7,242,657,716 | 10% | ||
| investprosper | 0 | 3,599,783,441 | 5.5% | ||
| lemony-cricket | 0 | 51,101,169,691 | 5.5% | ||
| miguelangel2801 | 0 | 780,545,630 | 50% | ||
| fantasycrypto | 0 | 1,043,956,088 | 0.65% | ||
| didic | 0 | 541,567,046 | 0.65% | ||
| emiliomoron | 0 | 18,197,784,005 | 50% | ||
| beverages | 0 | 2,045,789,956 | 0.65% | ||
| verhp11 | 0 | 1,116,331,347 | 1% | ||
| oghie | 0 | 734,010,876 | 50% | ||
| photohunt | 0 | 1,523,660,523 | 1.3% | ||
| geopolis | 0 | 610,953,124 | 10% | ||
| chungsu1 | 0 | 5,525,163,645 | 0.65% | ||
| robertbira | 0 | 1,036,571,114 | 2.5% | ||
| blervin | 0 | 6,518,179,112 | 11% | ||
| anikys3reasure | 0 | 548,174,303 | 10% | ||
| barge | 0 | 1,306,486,615 | 0.65% | ||
| thebluewin | 0 | 16,535,132,174 | 11% | ||
| alexdory | 0 | 11,921,195,844 | 10% | ||
| auminda | 0 | 23,529,629,373 | 11% | ||
| takowi | 0 | 17,097,392,189 | 1.3% | ||
| flugschwein | 0 | 3,869,428,364 | 8.5% | ||
| lightflares | 0 | 1,267,249,004 | 0.65% | ||
| doikao | 0 | 3,977,767,339 | 1.3% | ||
| francostem | 0 | 1,318,834,017 | 10% | ||
| endopediatria | 0 | 691,993,610 | 20% | ||
| forester-joe | 0 | 685,432,101 | 1.5% | ||
| vicesrus | 0 | 1,678,185,372 | 0.65% | ||
| croctopus | 0 | 1,462,234,884 | 100% | ||
| zipporah | 0 | 616,634,349 | 0.26% | ||
| idkpdx | 0 | 50,485,252 | 0.65% | ||
| superlotto | 0 | 1,674,300,344 | 1.3% | ||
| frassman | 0 | 783,827,381 | 5% | ||
| norwegianbikeman | 0 | 6,361,401,259 | 5% | ||
| satren | 0 | 32,124,156,693 | 10% | ||
| bscrypto | 0 | 2,296,065,052 | 0.65% | ||
| azircon | 0 | 1,515,295,810,322 | 9.35% | ||
| tomastonyperez | 0 | 16,735,446,791 | 50% | ||
| marcus0alameda | 0 | 922,942,861 | 50% | ||
| elvigia | 0 | 10,911,412,358 | 50% | ||
| camiloferrua | 0 | 539,950,574 | 0.71% | ||
| sanderjansenart | 0 | 621,284,062 | 0.65% | ||
| louis88 | 0 | 37,740,234,892 | 3.3% | ||
| qberry | 0 | 640,268,841 | 0.65% | ||
| lesmouths-travel | 0 | 553,166,282 | 7.5% | ||
| braaiboy | 0 | 2,108,129,271 | 0.97% | ||
| tonimontana | 0 | 124,106,123 | 5.76% | ||
| eniolw | 0 | 6,455,064,334 | 100% | ||
| de-stem | 0 | 5,713,497,356 | 9.9% | ||
| tijntje | 0 | 903,063,466 | 5.5% | ||
| taldor | 0 | 577,139,144 | 3.3% | ||
| josedelacruz | 0 | 8,969,123,475 | 50% | ||
| joseangelvs | 0 | 558,097,749 | 100% | ||
| kgakakillerg | 0 | 20,737,419,483 | 10% | ||
| payroll | 0 | 400,201,541,469 | 2% | ||
| mariusfebruary | 0 | 3,254,926,781 | 0.52% | ||
| hansdewet | 0 | 630,577,137 | 1.3% | ||
| saboin | 0 | 28,734,940,353 | 3.4% | ||
| meanbees | 0 | 17,720,858,487 | 5% | ||
| incubot | 0 | 1,286,972,177 | 0.97% | ||
| deholt | 0 | 531,912,723 | 8.5% | ||
| gwilberiol | 0 | 2,261,435,608 | 1.17% | ||
| temitayo-pelumi | 0 | 737,718,077 | 10% | ||
| andrick | 0 | 848,707,113 | 50% | ||
| yusvelasquez | 0 | 5,292,092,723 | 50% | ||
| motherofalegend | 0 | 1,114,831,911 | 5% | ||
| musicvoter2 | 0 | 4,373,455,617 | 1% | ||
| itastem | 0 | 3,564,895,832 | 10% | ||
| wolfofnostreet | 0 | 1,080,611,329 | 5.5% | ||
| uche-nna | 0 | 1,148,298,955 | 1.04% | ||
| lightcaptured | 0 | 1,203,854,554 | 0.65% | ||
| anaestrada12 | 0 | 19,069,949,846 | 100% | ||
| remotehorst23 | 0 | 17,334,811,785 | 11% | ||
| blewitt | 0 | 1,471,002,706 | 0.06% | ||
| frugal-fun | 0 | 3,722,823,244 | 100% | ||
| urdreamscometrue | 0 | 24,932,874,644 | 100% | ||
| bflanagin | 0 | 747,043,490 | 0.65% | ||
| ubaldonet | 0 | 2,899,232,332 | 70% | ||
| goblinknackers | 0 | 151,668,681,345 | 4% | ||
| zuerich | 0 | 452,170,484,727 | 10% | ||
| kylealex | 0 | 4,563,739,018 | 10% | ||
| loveforlove | 0 | 849,574,468 | 10% | ||
| voxmortis | 0 | 1,352,227,009 | 0.55% | ||
| spoke | 0 | 2,196,375,442 | 1.04% | ||
| fran.frey | 0 | 4,132,302,662 | 50% | ||
| nsfw-power | 0 | 13,761,765,587 | 11% | ||
| jrevilla | 0 | 637,359,909 | 100% | ||
| pboulet | 0 | 1,775,673,994 | 5% | ||
| marcocasario | 0 | 2,061,579,098 | 0.65% | ||
| stem-espanol | 0 | 69,705,694,330 | 100% | ||
| laissez-faire | 0 | 32,723,490 | 100% | ||
| the.success.club | 0 | 536,930,962 | 0.65% | ||
| macoolette | 0 | 22,776,374,378 | 3.3% | ||
| javier.dejuan | 0 | 1,311,105,314 | 10% | ||
| jmkengineering | 0 | 3,207,888,603 | 10% | ||
| meanroosterfarm | 0 | 2,023,906,806 | 5% | ||
| scienze | 0 | 4,464,376,311 | 10% | ||
| scienza | 0 | 4,510,371,074 | 10% | ||
| brianoflondon | 0 | 2,592,783,467 | 0.19% | ||
| giulyfarci52 | 0 | 1,681,086,346 | 50% | ||
| everyoung | 0 | 563,497,952 | 100% | ||
| sirpee6 | 0 | 4,529,401,394 | 100% | ||
| milky-concrete | 0 | 18,578,413,028 | 5.5% | ||
| adalger | 0 | 15,178,005,216 | 1.1% | ||
| cakemonster | 0 | 1,437,092,861 | 1.3% | ||
| the-rhapsodist | 0 | 9,656,035,367 | 10% | ||
| stem.witness | 0 | 20,847,630,366 | 10% | ||
| dein-problem | 0 | 0 | -0.04% | ||
| empressteemah | 0 | 538,186,951 | 10% | ||
| edriseur | 0 | 563,074 | 10% | ||
| robmojo | 0 | 902,667,694 | 0.55% | ||
| wilmer14molina | 0 | 3,981,274,809 | 50% | ||
| vaultec | 0 | 3,070,406,201 | 12% | ||
| crowdwitness | 0 | 8,815,148,701 | 5% | ||
| hairgistix | 0 | 611,834,559 | 0.65% | ||
| rem-steem | 0 | 650,317,650 | 0.26% | ||
| limka | 0 | 34,122,320 | 100% | ||
| cryptofiloz | 0 | 2,717,803,413 | 1.3% | ||
| epicdice | 0 | 24,437,851,669 | 3.3% | ||
| robibasa | 0 | 11,229,437,630 | 10% | ||
| scholaris | 0 | 15,311,748,896 | 2.5% | ||
| yourfuture | 0 | 880,653,541 | 7% | ||
| fractalfrank | 0 | 1,612,349,100 | 0.65% | ||
| likwid | 0 | 8,179,427,685 | 0.11% | ||
| tinyhousecryptos | 0 | 530,929,413 | 5% | ||
| kgswallet | 0 | 541,587,796 | 20% | ||
| xawi-ag | 0 | 536,131,569 | 15% | ||
| neoxiancity | 0 | 15,403,448,895 | 20% | ||
| good.game | 0 | 3,484,891,417 | 11% | ||
| babytarazkp | 0 | 573,724,424 | 10% | ||
| capp | 0 | 12,196,132,987 | 50% | ||
| curangel | 0 | 6,719,566,012,815 | 11% | ||
| vxc.stem | 0 | 0 | 5.76% | ||
| urtrailer | 0 | 1,388,569,048 | 0.5% | ||
| joshmania | 0 | 6,034,852,947 | 5.5% | ||
| steemstem-trig | 0 | 727,834,812 | 10% | ||
| herzinfuck | 0 | 537,152,341 | 0.65% | ||
| axel-blaze | 0 | 1,927,745,192 | 0.13% | ||
| sandymeyer | 0 | 10,460,106,547 | 4.4% | ||
| riccc96 | 0 | 31,562,880,068 | 100% | ||
| ibt-survival | 0 | 38,625,122,774 | 10% | ||
| yourtop3 | 0 | 1,950,667,432 | 0.52% | ||
| spinvest-neo | 0 | 542,679,449 | 10% | ||
| delilhavores | 0 | 3,635,445,558 | 20% | ||
| pavelsku | 0 | 4,625,809 | 0.65% | ||
| roamingsparrow | 0 | 990,015,764 | 0.97% | ||
| jeffmackinnon | 0 | 1,935,735,184 | 10% | ||
| juanvegetarian | 0 | 983,950,627 | 2.75% | ||
| pummeluff | 0 | 5,519,359,670 | 11% | ||
| dpend.active | 0 | 3,831,351,666 | 2.2% | ||
| risingstargame | 0 | 3,199,378,246 | 0.65% | ||
| fengchao | 0 | 3,684,534,797 | 3% | ||
| hornetsnest | 0 | 934,058,764 | 0.65% | ||
| stemsocial | 0 | 1,986,463,378 | 10% | ||
| hive.consultant | 0 | 1,059,997,947 | 1.04% | ||
| the100 | 0 | 587,493,742 | 0.65% | ||
| meppij | 0 | 640,751,489 | 0.19% | ||
| hiveonboard | 0 | 1,091,274,435 | 1.23% | ||
| arenacrypto | 0 | 901,636,364 | 5.5% |
Great! I love chemistry <3
| author | delilhavores |
|---|---|
| permlink | qensej |
| category | hive-196387 |
| json_metadata | {"app":"hiveblog/0.1"} |
| created | 2020-08-06 20:18:18 |
| last_update | 2020-08-06 20:18:18 |
| depth | 1 |
| children | 0 |
| last_payout | 2020-08-13 20:18:18 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 26 |
| author_reputation | 81,936,041,303,710 |
| root_title | "ISOMERISM IN ORGANIC COMPOUNDS" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 98,932,211 |
| net_rshares | 0 |
Congratulations @sirpee6! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) : <table><tr><td><img src="https://images.hive.blog/60x70/http://hivebuzz.me/@sirpee6/posts.png?202008041329"></td><td>You published more than 70 posts. Your next target is to reach 80 posts.</td></tr> </table> <sub>_You can view [your badges on your board](https://hivebuzz.me/@sirpee6) And compare to others on the [Ranking](https://hivebuzz.me/ranking)_</sub> <sub>_If you no longer want to receive notifications, reply to this comment with the word_ `STOP`</sub> **Do not miss the last post from @hivebuzz:** <table><tr><td><a href="/hivebuzz/@hivebuzz/pudresponse"><img src="https://images.hive.blog/64x128/https://i.imgur.com/805FIIt.jpg"></a></td><td><a href="/hivebuzz/@hivebuzz/pudresponse">Feedback from the last Hive Power Up Day</a></td></tr><tr><td><a href="/hivebuzz/@hivebuzz/pud"><img src="https://images.hive.blog/64x128/https://i.imgur.com/805FIIt.jpg"></a></td><td><a href="/hivebuzz/@hivebuzz/pud">Hive Power Up Day - Let's grow together!</a></td></tr></table>
| author | hivebuzz |
|---|---|
| permlink | hivebuzz-notify-sirpee6-20200804t134450000z |
| category | hive-196387 |
| json_metadata | {"image":["http://hivebuzz.me/notify.t6.png"]} |
| created | 2020-08-04 13:44:48 |
| last_update | 2020-08-04 13:44:48 |
| depth | 1 |
| children | 0 |
| last_payout | 2020-08-11 13:44:48 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 1,120 |
| author_reputation | 367,848,027,147,051 |
| root_title | "ISOMERISM IN ORGANIC COMPOUNDS" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 98,888,565 |
| net_rshares | 0 |
<div class='text-justify'> <div class='pull-left'> <img src='https://stem.openhive.network/images/stemsocialsupport7.png'> </div> Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us! Please consider <a href="https://hivesigner.com/sign/update-proposal-votes?proposal_ids=%5B91%5D&approve=true">supporting our funding proposal</a>, <a href="https://hivesigner.com/sign/account_witness_vote?approve=1&witness=stem.witness">approving our witness</a> (@stem.witness) or delegating to the @stemsocial account (for some ROI). Please consider using the <a href='https://stem.openhive.network'>STEMsocial app</a> app and including @stemsocial as a beneficiary to get a stronger support. <br /> <br />
| author | steemstem |
|---|---|
| permlink | re-sirpee6-isomerism-in-organic-compounds-20200805t160614712z |
| category | hive-196387 |
| json_metadata | {"app":"stemsocial"} |
| created | 2020-08-05 16:06:15 |
| last_update | 2020-08-05 16:06:15 |
| depth | 1 |
| children | 0 |
| last_payout | 2020-08-12 16:06:15 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 778 |
| author_reputation | 262,017,435,115,313 |
| root_title | "ISOMERISM IN ORGANIC COMPOUNDS" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 98,909,205 |
| net_rshares | 0 |
 hiveblocks
hiveblocks