Viewing a response to: @mikewick77/h5r2n
2,4-dimethyl-3-isopropylpentane Molecular Formula C10H22 https://pubchem.ncbi.nlm.nih.gov/compound/2_4-Dimethyl-3-isopropylpentane https://youtu.be/QaU7_hyFXrw .. guanidinium ion https://en.m.wikipedia.org/wiki/Guanidine https://en.m.wikipedia.org/wiki/Arginine https://en.m.wikipedia.org/wiki/Hydrogen_bond https://en.m.wikipedia.org/wiki/Nitric_oxide .. CHON carbon hydrogen oxygen nitrogen https://en.m.wikipedia.org/wiki/CHON https://en.m.wikipedia.org/wiki/Microbial_metabolism https://en.m.wikipedia.org/wiki/Methanogenesis https://en.m.wikipedia.org/wiki/Hydrogen_cycle https://en.m.wikipedia.org/wiki/Hydrogen_oxidizing_bacteria https://en.m.wikipedia.org/wiki/Hydrogenase https://en.m.wikipedia.org/wiki/Chlorine_dioxide https://en.m.wikipedia.org/wiki/Organochloride .. Chlorocarbon Organochloride Chlorinated Hydrocarbon Scientists finally crack nature’s most common chemical bond https://news.berkeley.edu/2020/05/21/scientists-finally-crack-natures-most-common-chemical-bond/ https://bio.libretexts.org/Courses/Northwest_University/MKBN211%3A_Introductory_Microbiology_(Bezuidenhout)/06%3A_Culturing_Microorganisms/6.01%3A_Microbial_Nutrition/6.1.02%3A_Sources_of_Essential_Nutrients .. i do believe the salt acid chlorine dioxide is a component to this puzzle. this article is talking about boron, which can be manufactured. looking for a connection between chlorine dioxide boron. i am convinced that Hydrogen Oxidizing Bacteria manufacture millions of hydrocarbon chemical arrangements. its basically jyst adding salt minerals to acid, breaks large bonds & forms ions. just need to be careful using strong acids & bases.. the results become dangerously caustic.. fermentation is safer because they live inside the chemical formula. manufacture only weak acids. what i read about the multiple oxides types of boron, they must be heated up. so.. if the weak acids of fermentation were boiled in salt minerals.. it would pasteurize the microbes & enhance the ions hydrocarbons & ionized oxides ect.. are made quickly threw weak acids, heat & salt minerals. so.. to bypass fermentation concerns, i wonder if white vinegar & multi mineral complex (including sodum) in low heat would manufacture similar compounds for cellular respiration & detox? very specifically no strong bases.. such as baking soda, very dangerous. very small amounts of mineral salts, a little goes a long way. a little carbon might be required.
| author | mikewick77 |
|---|---|
| permlink | qfc21d |
| category | mikewick77 |
| json_metadata | {"app":"hiveblog/0.1","image":["https://img.youtube.com/vi/QaU7_hyFXrw/0.jpg"],"links":["https://pubchem.ncbi.nlm.nih.gov/compound/2_4-Dimethyl-3-isopropylpentane","https://youtu.be/QaU7_hyFXrw","https://en.m.wikipedia.org/wiki/Guanidine","https://en.m.wikipedia.org/wiki/Arginine","https://en.m.wikipedia.org/wiki/Hydrogen_bond","https://en.m.wikipedia.org/wiki/Nitric_oxide","https://en.m.wikipedia.org/wiki/CHON","https://en.m.wikipedia.org/wiki/Microbial_metabolism","https://en.m.wikipedia.org/wiki/Methanogenesis","https://en.m.wikipedia.org/wiki/Hydrogen_cycle","https://en.m.wikipedia.org/wiki/Hydrogen_oxidizing_bacteria","https://en.m.wikipedia.org/wiki/Hydrogenase","https://en.m.wikipedia.org/wiki/Chlorine_dioxide","https://en.m.wikipedia.org/wiki/Organochloride","https://news.berkeley.edu/2020/05/21/scientists-finally-crack-natures-most-common-chemical-bond/","https://bio.libretexts.org/Courses/Northwest_University/MKBN211%3A_Introductory_Microbiology_"]} |
| created | 2020-08-19 22:48:51 |
| last_update | 2020-08-21 03:11:54 |
| depth | 1 |
| children | 1 |
| last_payout | 2020-08-26 22:48:51 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 2,469 |
| author_reputation | 545,063,717,246 |
| root_title | "Beam Me Up" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 99,163,523 |
| net_rshares | 4,822,455,416 |
| author_curate_reward | "" |
| voter | weight | wgt% | rshares | pct | time |
|---|---|---|---|---|---|
| mikewick77 | 0 | 4,822,455,416 | 100% |
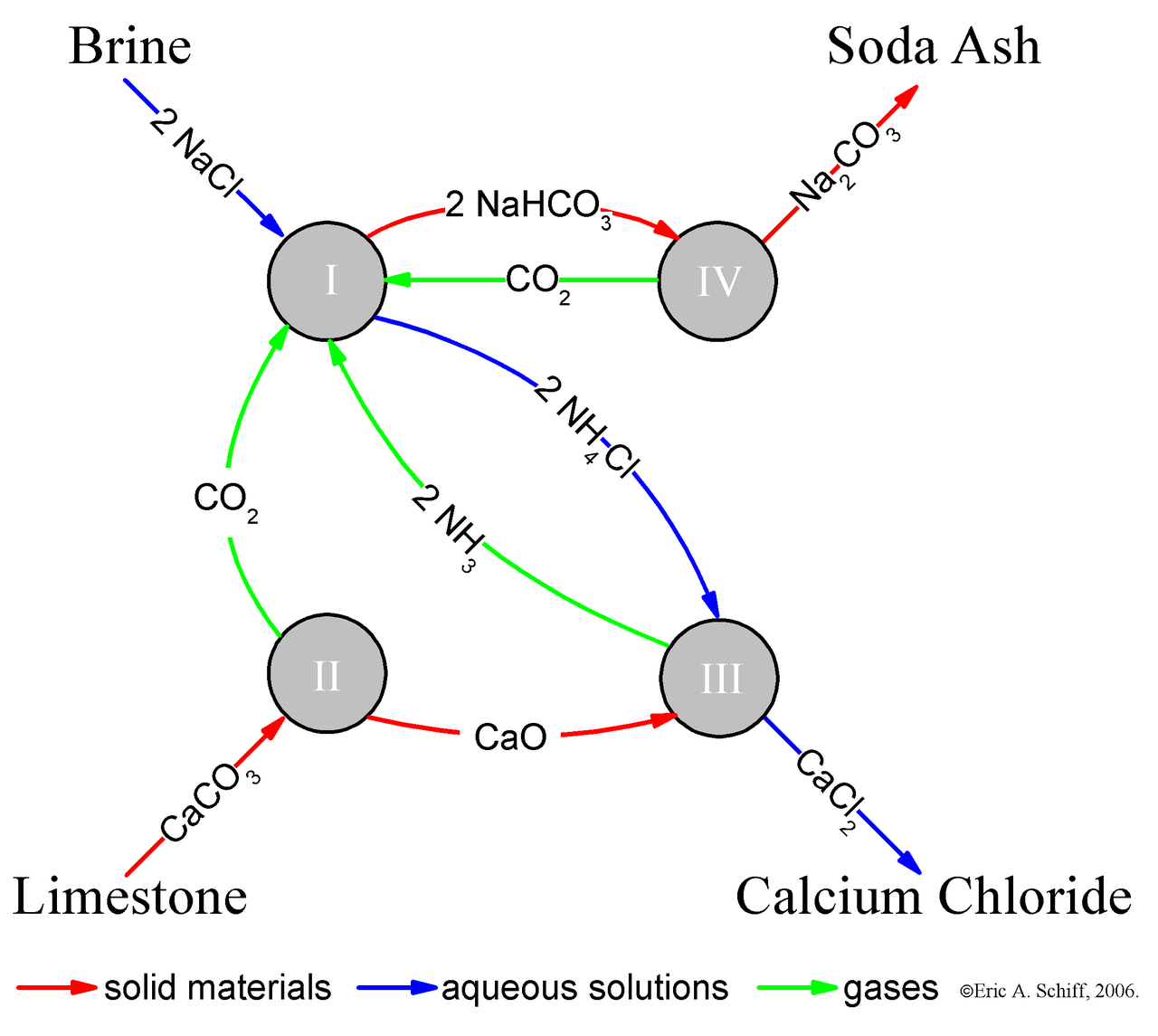 https://en.m.wikipedia.org/wiki/Mass_(mass_spectrometry) https://en.m.wikipedia.org/wiki/Nitrogen_rule https://en.m.wikipedia.org/wiki/Azide https://en.m.wikipedia.org/wiki/Ionic_compound https://en.m.wikipedia.org/wiki/Chemical_decomposition https://en.m.wikipedia.org/wiki/Sodium_carbonate https://en.m.wikipedia.org/wiki/Metalloprotein https://en.m.wikipedia.org/wiki/Amino_acid_synthesis https://en.m.wikipedia.org/wiki/Amine https://en.m.wikipedia.org/wiki/Carboxylic_acid https://en.m.wikipedia.org/wiki/Phosphodiester_bond .. amino acid synthesis chemical decomposition metalloprotein amino acid (protein) amine (hydrocarbon) carboxyl (acid) metal ion (mineral salt) CHON: carbon hydrogen oxygen nitrogen potash (potassium) phosphate (acid) .. Amino acids are organic compounds that contain amine (-NH2) and carboxyl (-COOH). Elements of an amino acid are carbon (C), hydrogen (H), oxygen(O), and nitrogen. Proteins are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Metalloprotein is a generic term for a protein that contains a metal ion cofactor.
| author | mikewick77 |
|---|---|
| permlink | qfgjxm |
| category | mikewick77 |
| json_metadata | {"links":["https://en.m.wikipedia.org/wiki/Mass_","https://en.m.wikipedia.org/wiki/Nitrogen_rule","https://en.m.wikipedia.org/wiki/Azide","https://en.m.wikipedia.org/wiki/Ionic_compound","https://en.m.wikipedia.org/wiki/Chemical_decomposition","https://en.m.wikipedia.org/wiki/Sodium_carbonate","https://en.m.wikipedia.org/wiki/Metalloprotein","https://en.m.wikipedia.org/wiki/Amino_acid_synthesis","https://en.m.wikipedia.org/wiki/Amine","https://en.m.wikipedia.org/wiki/Carboxylic_acid","https://en.m.wikipedia.org/wiki/Phosphodiester_bond"],"app":"hiveblog/0.1","image":["https://images.hive.blog/DQmSthEtLR7W9uouJf26vBQELgFws5uSgL5pJFCThB9ywSk/1280px-Solvay_Process.PNG.png"]} |
| created | 2020-08-22 09:05:48 |
| last_update | 2020-08-22 17:05:00 |
| depth | 2 |
| children | 0 |
| last_payout | 2020-08-29 09:05:48 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 1,273 |
| author_reputation | 545,063,717,246 |
| root_title | "Beam Me Up" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 99,207,249 |
| net_rshares | 0 |
 hiveblocks
hiveblocks