Hello everyone i hope you all are fine. In my last post i have discussed about the basic design of thermodynamics and zeroth law of thermodynamics. In this lecture or post i will tell you about the microscopic and macroscopic approach of studying thermodynamics, Equilibrium and about the adabatic wall and diathermal wall. .png) ***what is microscopic and macroscopic approach of thermodynamics*** In Microscopic approach of studying the system we study at the molecular level. We study each molecule in the system and the total change in any of the property is equals to the summation of all the changes. In microscopic approach each particle is study alone one by one. Microscopic approach is also known as statistical thermodynamics. In macroscopic approach a certain quantity of matter is considered without studying the system at the molecular level. In macroscopic view any change happen in the system is considered not how change takes place in the system example pressure we can't go to the molecular level how pressure is increasing we just consider how much pressure is increased or decreased. Macroscopic approach is also known as clasical thermodynamics. ***what is equilibrium*** A system is said to be in the equilibrium when there is not change in the system either at microscopic ot macroscopic level when the system is isolated from its surroundings. When the system is in equilibrium there no no physical chang in the system, system can not change from one state to the other. If you have to choose where the system is in equilibrium system must posses these three conditions or these three equilibriums: 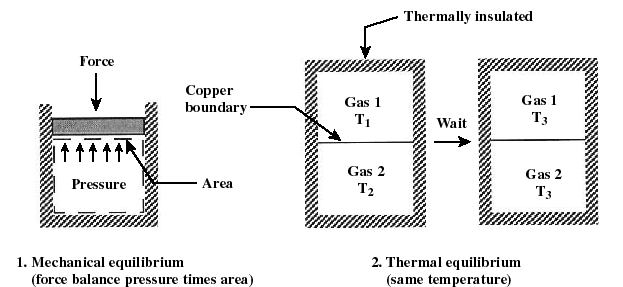.png) 1) mechanical equilibrium: A system is said to be in mechanical equilibrium when there in no unbalanced force between the system and the surrounding and with in the system. If there is an unbalanced force the system will get change of state. So it is easy to predict where the system is in mechanical equilibrium or not. 2) chemical equilibrium: A system is said to be in chemical equilibrium when there is no chemical change in the system or no chemical reaction takes place in the system and when there is no mass transfer from the system. 3) Thermal equilibrium: when the system satisfies the above two equilibriums the system is separated from its surroundings by the diathermal wall if there is no change in any of the property of the system the system is in thermal equilibrium. In thermal equilibrium the temperature of the system and the surrounding are same. If system satisfies these all three conditions then you can say the system is in equilibrium condition. 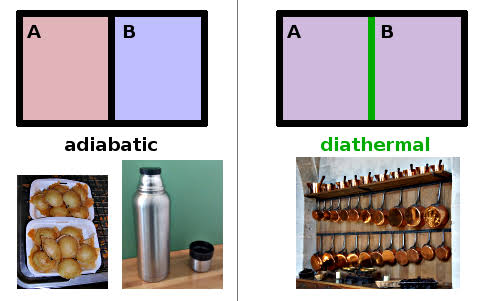.jpeg) ***What is adiabatic and diathermal wall*** Adiabatic wall is that wall that didn't allow heat transfer across it. Diathermal wall is that which allows the heat transfer across its boundary.
| author | alexcarlos |
|---|---|
| permlink | equilibrium-in-thermodynamics-and-some-small-concepts |
| category | hive-122108 |
| json_metadata | {"tags":["hive-122108","education","education-colmena","palnet","hiveisalive","hive","thermodynamics","hivestem"],"image":["https://cdn.steemitimages.com/DQmRhvboHtpMyo2qHcDdSuKCbkh58GYEFkoNCWZddUKHKZ8/images%20(3).png","https://cdn.steemitimages.com/DQmddA4Yirc651pm2RwAwe371icdzkZkSkMFjzFeWNZqswG/images%20(2).png","https://cdn.steemitimages.com/DQmUfyY5mMmArT7CnnbVyqawX7vdwcpFprT6gPMRhmCFNyJ/images%20(14).jpeg"],"app":"hiveblog/0.1","format":"markdown"} |
| created | 2020-04-13 18:30:06 |
| last_update | 2020-04-13 18:30:06 |
| depth | 0 |
| children | 1 |
| last_payout | 2020-04-20 18:30:06 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 2.181 HBD |
| curator_payout_value | 2.149 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 3,171 |
| author_reputation | 8,605,657,766,341 |
| root_title | "Equilibrium in thermodynamics and some small concepts" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 96,827,473 |
| net_rshares | 13,784,552,546,453 |
| author_curate_reward | "" |
| voter | weight | wgt% | rshares | pct | time |
|---|---|---|---|---|---|
| tombstone | 0 | 144,477,431,662 | 0.56% | ||
| tuck-fheman | 0 | 915,619,575 | 4.5% | ||
| chris4210 | 0 | 41,903,431,277 | 4.5% | ||
| kevinwong | 0 | 258,456,726,560 | 3.15% | ||
| justtryme90 | 0 | 195,960,903,572 | 4.5% | ||
| eric-boucher | 0 | 18,997,551,271 | 4.5% | ||
| thecryptodrive | 0 | 20,108,389,599 | 1.8% | ||
| anwenbaumeister | 0 | 416,417,449 | 9% | ||
| mammasitta | 0 | 3,282,525,804 | 0.45% | ||
| matt-a | 0 | 24,293,660,103 | 1.5% | ||
| redpalestino | 0 | 73,976,763,134 | 1.57% | ||
| mrwang | 0 | 1,025,201,866 | 1.57% | ||
| blueorgy | 0 | 22,417,169,588 | 6.75% | ||
| arconite | 0 | 1,075,568,514 | 1.57% | ||
| kibela | 0 | 731,866,328 | 2.7% | ||
| eztechwin | 0 | 3,913,147,674 | 4.5% | ||
| psygambler | 0 | 826,682,773 | 4.5% | ||
| djennyfloro | 0 | 1,308,679,202 | 10% | ||
| randomblock1 | 0 | 1,419,422,133 | 9% | ||
| hanshotfirst | 0 | 2,423,916,154,203 | 50% | ||
| lordvader | 0 | 933,108,076,439 | 100% | ||
| azizbd | 0 | 21,464,708,797 | 9% | ||
| uwelang | 0 | 60,966,552,102 | 4.5% | ||
| holoz0r | 0 | 109,630,802,449 | 18% | ||
| lk666 | 0 | 4,421,902,351 | 4.5% | ||
| curie | 0 | 906,287,851,475 | 9% | ||
| modernzorker | 0 | 3,491,936,864 | 6.3% | ||
| techslut | 0 | 129,108,912,087 | 25% | ||
| hendrikdegrote | 0 | 111,622,162,710 | 9% | ||
| vact | 0 | 1,198,732,485 | 9% | ||
| giantbear | 0 | 6,544,485,388 | 9% | ||
| roguewriter | 0 | 1,144,105,697 | 4.5% | ||
| dashfit | 0 | 1,008,058,006 | 4.5% | ||
| tristancarax | 0 | 6,274,492,084 | 9% | ||
| sweetpea | 0 | 7,372,407,758 | 50% | ||
| apsu | 0 | 5,104,517,596 | 3.15% | ||
| nutman | 0 | 121,950,638 | 9% | ||
| driptorchpress | 0 | 2,714,786,977 | 4.5% | ||
| gmedley | 0 | 1,936,056,250 | 4.5% | ||
| theregularguy | 0 | 168,963,662,613 | 100% | ||
| steemiteducation | 0 | 660,114,298,329 | 90% | ||
| dhimmel | 0 | 126,592,844,694 | 3.15% | ||
| alinabarbu | 0 | 1,782,408,764 | 20% | ||
| bloom | 0 | 19,266,727,271 | 4.5% | ||
| federacion45 | 0 | 4,267,447,307 | 4.5% | ||
| arnel | 0 | 2,029,353,000 | 4.5% | ||
| iansart | 0 | 9,950,126,973 | 4.5% | ||
| forykw | 0 | 20,033,942,524 | 4.5% | ||
| jagged | 0 | 5,707,888,747 | 1.8% | ||
| bitrocker2020 | 0 | 25,600,004,876 | 1.8% | ||
| sustainablyyours | 0 | 3,400,423,078 | 4.5% | ||
| yehey | 0 | 126,916,335,285 | 9% | ||
| freetissues | 0 | 8,238,824,849 | 4.5% | ||
| cindycam | 0 | 1,967,890,790 | 50% | ||
| deisip67 | 0 | 1,100,165,443 | 20% | ||
| schoolforsdg4 | 0 | 9,737,073,559 | 5% | ||
| wishmaiden | 0 | 1,248,428,898 | 4.5% | ||
| mxzn | 0 | 5,747,741,876 | 4.5% | ||
| roseri | 0 | 3,541,020,788 | 50% | ||
| zerotoone | 0 | 1,832,601,229 | 4.5% | ||
| locikll | 0 | 3,533,652,425 | 18% | ||
| kalinka | 0 | 1,792,669,679 | 4.5% | ||
| mahdiyari | 0 | 35,287,615,498 | 9% | ||
| aboutyourbiz | 0 | 1,925,942,419 | 9% | ||
| oscarcede | 0 | 785,623,964 | 18% | ||
| ghostgtr | 0 | 1,913,915,818 | 18% | ||
| jayna | 0 | 5,287,075,656 | 1.8% | ||
| guchtere | 0 | 840,906,613 | 4.5% | ||
| rival | 0 | 3,421,584,582 | 2% | ||
| wesphilbin | 0 | 625,179,325 | 1.8% | ||
| affiedalfayed | 0 | 4,238,541,649 | 36% | ||
| gunthertopp | 0 | 1,858,745,496,411 | 45% | ||
| binkyprod | 0 | 2,375,281,555 | 4.5% | ||
| tngflx | 0 | 1,553,729,512 | 2.7% | ||
| flatman | 0 | 8,905,904,311 | 9% | ||
| allcapsonezero | 0 | 3,581,263,565 | 4.5% | ||
| minnowbooster | 0 | 278,251,893,134 | 1.8% | ||
| felt.buzz | 0 | 4,940,251,669 | 2.25% | ||
| steemwizards | 0 | 69,299,893,609 | 100% | ||
| neumannsalva | 0 | 2,778,069,152 | 4.5% | ||
| stayoutoftherz | 0 | 106,860,537,264 | 4.5% | ||
| ralph-rennoldson | 0 | 1,173,652,458 | 1.6% | ||
| jimshorts | 0 | 9,287,078,894 | 100% | ||
| supriya1993 | 0 | 3,949,117,689 | 15% | ||
| prapanth | 0 | 1,914,798,838 | 4.5% | ||
| hdmed | 0 | 557,638,251 | 9% | ||
| dream.trip | 0 | 713,446,390 | 9% | ||
| guada1 | 0 | 924,253,628 | 45% | ||
| foreveraverage | 0 | 861,174,134 | 4.5% | ||
| garudi | 0 | 561,661,080 | 9% | ||
| steemorocco | 0 | 1,102,399,613 | 100% | ||
| revo | 0 | 41,843,824,111 | 9% | ||
| nerdnews | 0 | 16,816,000,260 | 100% | ||
| mulletwang | 0 | 11,755,201,600 | 35% | ||
| stickchumpion | 0 | 1,345,990,273 | 4.5% | ||
| noloafing | 0 | 5,054,164,393 | 2.25% | ||
| thelordsharvest | 0 | 8,698,661,388 | 9% | ||
| kimzwarch | 0 | 8,876,409,702 | 4% | ||
| olusolaemmanuel | 0 | 2,000,552,184 | 6.3% | ||
| massivevibration | 0 | 9,340,068,224 | 5% | ||
| fbslo | 0 | 13,004,414,510 | 9% | ||
| vera-vaders-ea | 0 | 8,861,547,522 | 100% | ||
| holbein81 | 0 | 36,950,847,474 | 7.65% | ||
| justinparke | 0 | 1,691,431,285 | 5% | ||
| torico | 0 | 2,008,752,523 | 2.97% | ||
| masterwu | 0 | 3,095,087,572 | 27% | ||
| yangoldberg | 0 | 36,263,222,098 | 9% | ||
| karaoke1850 | 0 | 44,382,312,197 | 100% | ||
| minnowpowerup | 0 | 2,013,973,109 | 4.5% | ||
| majes.tytyty | 0 | 21,517,844,037 | 1.8% | ||
| dokter-purnama | 0 | 874,888,946 | 4.5% | ||
| derekvonzarovich | 0 | 725,489,187 | 4.5% | ||
| cryptononymous | 0 | 1,140,470,570 | 4.5% | ||
| upme | 0 | 4,875,563,353 | 4.5% | ||
| mendoza | 0 | 602,332,016 | 9% | ||
| gotgame | 0 | 1,792,922,076 | 4.5% | ||
| braveboat | 0 | 2,637,600,502 | 8% | ||
| jlsplatts | 0 | 9,442,350,610 | 2% | ||
| dauerossi | 0 | 4,541,515,664 | 30% | ||
| buttcoins | 0 | 6,212,338,152 | 1.8% | ||
| gregan | 0 | 726,849,448 | 4.5% | ||
| divinekids | 0 | 7,467,354,924 | 100% | ||
| toocurious | 0 | 6,653,268,798 | 4.5% | ||
| hanggggbeeee | 0 | 1,403,635,809 | 4.5% | ||
| steemed-proxy | 0 | 932,424,914,870 | 4.5% | ||
| fatkat | 0 | 2,378,549,863 | 4.49% | ||
| morwhale | 0 | 2,500,738,654 | 100% | ||
| stevejhuggett | 0 | 10,114,004,839 | 20% | ||
| peaceandwar | 0 | 1,443,373,367 | 4.5% | ||
| tazbaz | 0 | 863,230,521 | 4.5% | ||
| teacherspet | 0 | 21,004,702,527 | 100% | ||
| morwhaleplus | 0 | 851,769,954 | 100% | ||
| battebilly | 0 | 1,385,601,987 | 4.5% | ||
| notb4mycoffee | 0 | 52,378,116,373 | 100% | ||
| silverwhale | 0 | 23,772,898,400 | 90% | ||
| alvinauh | 0 | 31,404,161,680 | 30% | ||
| morwhalebonus | 0 | 843,053,333 | 100% | ||
| teammorocco | 0 | 4,322,688,738 | 100% | ||
| wolfnworbeikood | 0 | 4,872,064,675 | 13% | ||
| feedme | 0 | 640,622,704 | 100% | ||
| bluefinstudios | 0 | 1,590,964,460 | 2.7% | ||
| steveconnor | 0 | 5,192,435,135 | 4.5% | ||
| sankysanket18 | 0 | 5,129,662,306 | 4.5% | ||
| misterbob | 0 | 801,591,075 | 45% | ||
| nicole-st | 0 | 2,916,146,628 | 4.5% | ||
| teukurival | 0 | 765,998,798 | 4.5% | ||
| jasimg | 0 | 40,786,951,021 | 100% | ||
| drmake | 0 | 5,018,110,206 | 4.5% | ||
| sarmitirajaa | 0 | 552,928,527 | 100% | ||
| blue-pencil | 0 | 12,968,780,854 | 90% | ||
| danile666 | 0 | 5,944,046,985 | 4.5% | ||
| pechichemena | 0 | 3,421,542,579 | 1.8% | ||
| seyiodus | 0 | 1,117,612,325 | 20% | ||
| citizensmith | 0 | 6,862,223,924 | 4.5% | ||
| afifa | 0 | 836,039,005 | 10% | ||
| mhm-philippines | 0 | 14,683,509,433 | 4.5% | ||
| hanzappedfirst | 0 | 71,449,441,829 | 100% | ||
| skycae | 0 | 1,197,601,616 | 9% | ||
| itchyfeetdonica | 0 | 6,206,478,745 | 1.8% | ||
| weirdnews | 0 | 8,179,418,156 | 100% | ||
| xanderslee | 0 | 701,831,933 | 9% | ||
| brutledge | 0 | 605,741,518 | 4.5% | ||
| kenadis | 0 | 1,188,416,969 | 4.5% | ||
| esaia.mystic | 0 | 578,817,152 | 9% | ||
| onemedia | 0 | 911,282,094 | 9% | ||
| iptrucs | 0 | 41,100,057,890 | 25% | ||
| punchline | 0 | 246,124,774,731 | 100% | ||
| lextenebris | 0 | 1,730,992,928 | 4.5% | ||
| thescubageek | 0 | 634,088,415 | 4.5% | ||
| najat | 0 | 3,179,617,282 | 100% | ||
| venalbe | 0 | 999,411,524 | 4.5% | ||
| danaedwards | 0 | 1,258,763,601 | 9% | ||
| phgnomo | 0 | 911,820,440 | 4.5% | ||
| redcube | 0 | 1,184,823,121 | 4.5% | ||
| gordon92 | 0 | 979,630,802 | 4.5% | ||
| stahlberg | 0 | 2,098,898,691 | 4.5% | ||
| reizak | 0 | 830,214,246 | 3.6% | ||
| crypto3lite | 0 | 2,548,676,694 | 80% | ||
| carn | 0 | 1,036,079,060 | 6.75% | ||
| iamjadeline | 0 | 2,222,920,139 | 1.35% | ||
| hijosdelhombre | 0 | 3,629,428,956 | 4.5% | ||
| hdmed.dev | 0 | 573,305,770 | 100% | ||
| soufianechakrouf | 0 | 1,729,228,705 | 2.7% | ||
| shinedojo | 0 | 1,203,106,088 | 9% | ||
| epic-fail | 0 | 9,546,253,065 | 4.5% | ||
| bennettitalia | 0 | 1,602,370,895 | 2.25% | ||
| utube | 0 | 3,866,541,971 | 9% | ||
| itestify | 0 | 490,667,345 | 30% | ||
| steveblucher | 0 | 5,091,778,885 | 45% | ||
| womenempowerment | 0 | 2,288,423,287 | 5% | ||
| kylo-ren | 0 | 7,623,138,503 | 100% | ||
| neneandy | 0 | 10,398,314,859 | 9% | ||
| shares | 0 | 4,262,697,726 | 9% | ||
| videosteemit | 0 | 14,593,670,446 | 9% | ||
| mashiliyanage | 0 | 1,303,695,959 | 45% | ||
| didic | 0 | 4,036,762,051 | 4.5% | ||
| warpedpoetic | 0 | 2,617,287,345 | 9% | ||
| operahoser | 0 | 833,531,039 | 1.35% | ||
| upfundme | 0 | 561,773,313 | 0.45% | ||
| beverages | 0 | 14,256,790,922 | 4.5% | ||
| nwjordan | 0 | 1,409,547,221 | 9% | ||
| sandracabrera | 0 | 2,532,859,088 | 45% | ||
| oghie | 0 | 2,108,472,061 | 50% | ||
| photohunt | 0 | 7,318,459,746 | 9% | ||
| ejlo3310 | 0 | 2,493,019,775 | 45% | ||
| chungsu1 | 0 | 127,139,953,794 | 4.5% | ||
| dacx | 0 | 22,884,945,958 | 8.1% | ||
| takowi | 0 | 59,751,077,936 | 9% | ||
| vegan.niinja | 0 | 625,846,332 | 4.5% | ||
| lightflares | 0 | 9,441,737,375 | 4.5% | ||
| cyprianj | 0 | 1,424,966,190 | 9% | ||
| bernardino | 0 | 548,364,394 | 4.5% | ||
| russellstockley | 0 | 1,007,762,836 | 10% | ||
| kitalee | 0 | 4,188,072,508 | 4.5% | ||
| forester-joe | 0 | 735,881,708 | 1.5% | ||
| vicesrus | 0 | 12,531,045,247 | 4.5% | ||
| steemitbingo | 0 | 782,221,029 | 4.5% | ||
| zipporah | 0 | 4,245,496,074 | 1.8% | ||
| nezer | 0 | 3,458,994,220 | 9% | ||
| hadley4 | 0 | 1,731,603,284 | 9% | ||
| superlotto | 0 | 18,087,717,932 | 9% | ||
| norwegianbikeman | 0 | 17,367,024,077 | 20% | ||
| ambitiouslife | 0 | 551,204,239 | 4.5% | ||
| positiveninja | 0 | 1,435,042,403 | 4.5% | ||
| deathlyhorror | 0 | 2,366,996,453 | 4.5% | ||
| miroslavrc | 0 | 4,698,220,498 | 2.25% | ||
| steemlibs | 0 | 616,263,385 | 4.5% | ||
| foxyspirit | 0 | 1,519,311,266 | 4.5% | ||
| bscrypto | 0 | 5,018,044,548 | 2.25% | ||
| bil.prag | 0 | 920,633,417 | 0.45% | ||
| camiloferrua | 0 | 4,630,171,053 | 4.95% | ||
| sanderjansenart | 0 | 4,202,162,824 | 4.5% | ||
| vittoriozuccala | 0 | 2,542,123,564 | 4.5% | ||
| qberry | 0 | 4,750,264,655 | 4.5% | ||
| frissonsteemit | 0 | 1,735,660,310 | 4.5% | ||
| stmdev | 0 | 218,116,352 | 2% | ||
| broncofan99 | 0 | 23,749,730,823 | 20% | ||
| rambutan.art | 0 | 2,389,686,482 | 9% | ||
| greddyforce | 0 | 6,321,136,354 | 4.05% | ||
| alanasteemit | 0 | 966,986,992 | 45% | ||
| flyerchen | 0 | 563,776,536 | 4.5% | ||
| c0wtschpotato | 0 | 862,837,387 | 4.5% | ||
| ilazramusic | 0 | 935,006,327 | 4.5% | ||
| misan | 0 | 501,712,026 | 4.5% | ||
| scruffy23 | 0 | 20,300,563,015 | 50% | ||
| misia1979 | 0 | 942,051,089 | 4.5% | ||
| payroll | 0 | 1,145,207,784,455 | 5% | ||
| dearw | 0 | 16,163,124,119 | 9% | ||
| outtheshellvlog | 0 | 1,318,243,257 | 4.5% | ||
| mraggaj | 0 | 1,471,568,596 | 5% | ||
| realblockchain | 0 | 4,854,152,235 | 20% | ||
| srijana-gurung | 0 | 1,803,041,390 | 4.5% | ||
| apteacher | 0 | 2,202,966,544 | 18% | ||
| jancharlest | 0 | 5,327,006,181 | 4.5% | ||
| musicvoter | 0 | 9,222,652,014 | 2% | ||
| kamalamezwar | 0 | 598,515,907 | 100% | ||
| netzisde | 0 | 30,601,750,272 | 9% | ||
| aceaeterna | 0 | 4,401,492,579 | 45% | ||
| pladozero | 0 | 74,979,872,614 | 10% | ||
| steemjet | 0 | 942,651,179 | 4.5% | ||
| motherofalegend | 0 | 1,259,632,935 | 4.5% | ||
| gracelbm | 0 | 1,018,929,351 | 4.5% | ||
| musicvoter2 | 0 | 8,389,101,570 | 1% | ||
| alrashel | 0 | 535,716,686 | 100% | ||
| gravii4 | 0 | 1,751,135,510 | 9% | ||
| myfreebtc | 0 | 3,750,014,841 | 6.3% | ||
| drawmeaship | 0 | 586,389,937 | 4.5% | ||
| lightcaptured | 0 | 1,150,327,381 | 4.5% | ||
| schroders | 0 | 3,584,376,137 | 2.7% | ||
| bububoomt | 0 | 542,998,645 | 9% | ||
| hardaeborla | 0 | 563,768,186 | 4.5% | ||
| melissaofficial | 0 | 5,544,153,643 | 9% | ||
| citizendog | 0 | 11,522,667,731 | 9% | ||
| council | 0 | 1,286,199,204 | 9% | ||
| cheese4ead | 0 | 1,808,559,178 | 4.5% | ||
| blewitt | 0 | 12,820,651,990 | 0.45% | ||
| leilanyarevalo | 0 | 543,504,245 | 30% | ||
| fernando.lubezki | 0 | 874,762,952 | 8.1% | ||
| ilovecryptopl | 0 | 1,516,606,518 | 7.2% | ||
| bflanagin | 0 | 5,412,013,112 | 4.5% | ||
| sadbear | 0 | 727,077,216 | 4.5% | ||
| call-me-howie | 0 | 849,769,813 | 4.5% | ||
| gerdtrudroepke | 0 | 1,321,358,408 | 2.25% | ||
| hansmast | 0 | 705,460,902 | 4.5% | ||
| goblinknackers | 0 | 129,689,417,895 | 4% | ||
| anttn | 0 | 85,656,263,770 | 30% | ||
| dronegraphica | 0 | 629,665,449 | 2.25% | ||
| honeycup-waters | 0 | 797,450,779 | 4.5% | ||
| pvinny69 | 0 | 2,475,443,735 | 9% | ||
| orthodoxnudism | 0 | 1,030,774,939 | 4.5% | ||
| kylealex | 0 | 5,669,119,358 | 10% | ||
| arnilarn | 0 | 628,518,015 | 9% | ||
| orlandogonzalez | 0 | 1,875,553,579 | 25% | ||
| minimining | 0 | 1,125,072,321 | 4.5% | ||
| spoke | 0 | 13,213,074,512 | 7.2% | ||
| edu-venezuela | 0 | 100,231,031,147 | 100% | ||
| hanshotfirst-sm | 0 | 7,797,391,776 | 100% | ||
| annaabi | 0 | 1,051,195,659 | 4.5% | ||
| marcocasario | 0 | 11,387,322,077 | 4.5% | ||
| trang | 0 | 686,460,128 | 4.5% | ||
| palasatenea | 0 | 1,010,818,596 | 4.5% | ||
| knightbjj | 0 | 1,521,664,252 | 6.75% | ||
| the.success.club | 0 | 4,100,270,726 | 4.5% | ||
| chickenmeat | 0 | 1,890,696,230 | 4.5% | ||
| macoolette | 0 | 34,966,800,120 | 2.7% | ||
| javier.dejuan | 0 | 2,714,331,644 | 9% | ||
| jmkengineering | 0 | 2,767,779,691 | 2.25% | ||
| tommyl33 | 0 | 1,187,250,046 | 4.5% | ||
| dubignyp | 0 | 1,350,306,384 | 50% | ||
| reverseacid | 0 | 818,319,371 | 4.5% | ||
| pushpedal | 0 | 880,039,648 | 4.5% | ||
| esthersanchez | 0 | 2,575,840,608 | 40% | ||
| alvin0617 | 0 | 823,209,233 | 4.5% | ||
| solarphasing | 0 | 696,247,465 | 5% | ||
| cakemonster | 0 | 14,669,391,826 | 9% | ||
| letalis-laetitia | 0 | 763,335,074 | 4.5% | ||
| double-negative | 0 | 580,447,084 | 20% | ||
| khan.dayyanz | 0 | 1,903,534,533 | 9% | ||
| ppss | 0 | 501,373,776 | 8.82% | ||
| michaias | 0 | 2,109,289,365 | 75% | ||
| healthexpert | 0 | 7,204,285,272 | 4.5% | ||
| vaultec | 0 | 5,010,226,734 | 12% | ||
| steemstorage | 0 | 3,810,247,367 | 9% | ||
| steemegg | 0 | 855,175,890 | 2.25% | ||
| travisung | 0 | 18,506,174,306 | 4.5% | ||
| loliver | 0 | 3,205,657,147 | 9% | ||
| hairgistix | 0 | 4,448,564,470 | 4.5% | ||
| rem-steem | 0 | 3,170,725,580 | 1.8% | ||
| pulleyhead | 0 | 2,552,985,029 | 9% | ||
| bluemaskman | 0 | 873,675,570 | 4.5% | ||
| cryptological | 0 | 1,185,762,913 | 4.5% | ||
| proxy-pal | 0 | 1,015,162,686 | 4.5% | ||
| extravagante | 0 | 1,753,442,579 | 100% | ||
| breakout101 | 0 | 1,077,317,327 | 4.5% | ||
| pedrobrito2004 | 0 | 3,284,905,697 | 9% | ||
| cryptofiloz | 0 | 22,171,377,788 | 9% | ||
| smalltall | 0 | 7,960,178,395 | 50% | ||
| jackramsey | 0 | 1,278,021,169 | 6.3% | ||
| filosof103 | 0 | 1,554,430,781 | 4.5% | ||
| begood2me | 0 | 797,350,543 | 4.5% | ||
| epicdice | 0 | 47,358,033,729 | 2.7% | ||
| deeanndmathews | 0 | 2,273,104,958 | 4.5% | ||
| edencourage | 0 | 6,391,838,060 | 50% | ||
| robibasa | 0 | 6,641,532,721 | 10% | ||
| newtrailers | 0 | 3,143,851,634 | 9% | ||
| socialbot | 0 | 7,173,223,425 | 5% | ||
| fractalfrank | 0 | 11,677,338,224 | 4.5% | ||
| ph1102 | 0 | 26,892,208,611 | 9% | ||
| waltermeth | 0 | 3,034,362,910 | 20% | ||
| titan-c | 0 | 4,619,128,096 | 9% | ||
| reggaesteem | 0 | 4,153,383,994 | 5% | ||
| drlobes | 0 | 810,735,103 | 4.5% | ||
| ufm.pay | 0 | 2,253,396,491 | 0.33% | ||
| tyrionlens | 0 | 19,487,133,854 | 100% | ||
| steem.consultant | 0 | 2,419,509,877 | 1.8% | ||
| andylein | 0 | 3,672,601,055 | 4.5% | ||
| behram | 0 | 9,663,228,468 | 50% | ||
| joshmania | 0 | 5,764,968,342 | 4.5% | ||
| writertales | 0 | 630,188,989 | 9% | ||
| ibt-survival | 0 | 40,034,747,752 | 10% | ||
| yourtop3 | 0 | 11,344,946,224 | 3.6% | ||
| roamingsparrow | 0 | 2,120,434,490 | 6.75% | ||
| appics.tutorial | 0 | 27,828,101,618 | 100% | ||
| reghunter | 0 | 1,492,912,591 | 25% | ||
| zirky | 0 | 2,611,030,189 | 60% | ||
| traveler-hwi | 0 | 1,696,265,207 | 4.5% | ||
| disagio.gang | 0 | 13,034,772,950 | 9% | ||
| highborn | 0 | 3,177,359,158 | 100% | ||
| drew0 | 0 | 679,624,619 | 4.5% | ||
| thecryptosociety | 0 | 17,329,641,428 | 25% | ||
| hive-naija | 0 | 1,251,685,638 | 4.5% | ||
| thepeakstudio | 0 | 4,283,145,383 | 4.5% | ||
| featheredfriend | 0 | 1,736,437,594 | 4.5% | ||
| hive.consultant | 0 | 918,779,714 | 4.5% |
Very informative !
| author | alrashel |
|---|---|
| permlink | q8r6pd |
| category | hive-122108 |
| json_metadata | {"app":"hiveblog/0.1"} |
| created | 2020-04-14 00:58:27 |
| last_update | 2020-04-14 00:58:27 |
| depth | 1 |
| children | 0 |
| last_payout | 2020-04-21 00:58:27 |
| cashout_time | 1969-12-31 23:59:59 |
| total_payout_value | 0.000 HBD |
| curator_payout_value | 0.000 HBD |
| pending_payout_value | 0.000 HBD |
| promoted | 0.000 HBD |
| body_length | 18 |
| author_reputation | 2,707,464,674,673 |
| root_title | "Equilibrium in thermodynamics and some small concepts" |
| beneficiaries | [] |
| max_accepted_payout | 1,000,000.000 HBD |
| percent_hbd | 10,000 |
| post_id | 96,831,513 |
| net_rshares | 0 |
 hiveblocks
hiveblocks